Open Label Clinical Trial
Open label study listen oh pen lay bel stuh dee a type of study in which both the health providers and the patients are aware of the drug or treatment being given. There are inherent differences between the two types of trials which both evaluate the safety and efficacy of investigational drugs and treatments.
 First Line Alk Mnsclc Clinical Trial Alecensa Alectinib
First Line Alk Mnsclc Clinical Trial Alecensa Alectinib
55 an open label trial of escitalopram for sad patients n 20 over 8 weeks produced a response rate of 95 sigh sad 50 of baseline value and a remission rate sigh sad score 8 of 85.

Open label clinical trial. Open label trials are sometimes referred to as non masked or unblinded if the trial is a non pharmacological study such as a trial of devices or psychological and physical treatments it may be referred to simply as open after recruitment to the trial the participants were allocated to treatment using block randomisation. An open label trial is a clinical trial conducted in a way that allows subjects and researchers to know which treatments are being used. The four phases of clinical research trials.
56 in an open label trial of. In an open label trial no attempt is made to disguise the investigational treatment and no placebo or control treatment is used. An open label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Open label trials of desipramine tranylcypromine reboxetine and bupropion showed improvement with the drug therapy. Open label clinical research trials. Without a placebo group with all participants receiving the same treatment.
There are typically two groups of clinical trials. Comparative studies and open label studies. As a result both physician and patient know which treatment is being provided.
What types of trials are there. The four phases of clinical research trials. This leans towards bias as both the patient and the physician are aware of which groups are receiving what type of treatment.
Open label clinical trials do not attempt to disguise the new drug or treatment meaning that no standard treatment or placebo is utilized. Open label clinical research trials. Sometimes the circumstances of a trial make it impossible to conceal the identity of the treatment patients are receiving and in other cases researchers may have a specific reason for wanting to use the open label trial format.
Open label trials may also be uncontrolled ie. The nci dictionary of cancer terms features 8360 terms related to cancer and medicine.
 Onivyde Irinotecan Liposome Injection Napoli 1 Study Design Hcp
Onivyde Irinotecan Liposome Injection Napoli 1 Study Design Hcp
Rationale And Design Of A Multi Center Open Label Randomised
 Open Label Clinical Trial Fresh Explaining The Jargon Clinical
Open Label Clinical Trial Fresh Explaining The Jargon Clinical
Gtl001 A Therapeutic Vaccine For Women Infected With Human
Late Stage Cancer Treatment Of Colorectal Cancer Oncology
Echo Study Evidence For Contraceptive Options Hiv Outcomes Echo
 Phase I Open Label Liver Directed Gene Therapy Clinical Trial For
Phase I Open Label Liver Directed Gene Therapy Clinical Trial For
 Catabasis Pharmaceuticals Catb Open Label Extension Eesults From
Catabasis Pharmaceuticals Catb Open Label Extension Eesults From
Isis Releases Encouraging Results From Phase 2 Study Cure Sma
 Open Label Extension Studies And The Ethical Design Of Clinical
Open Label Extension Studies And The Ethical Design Of Clinical
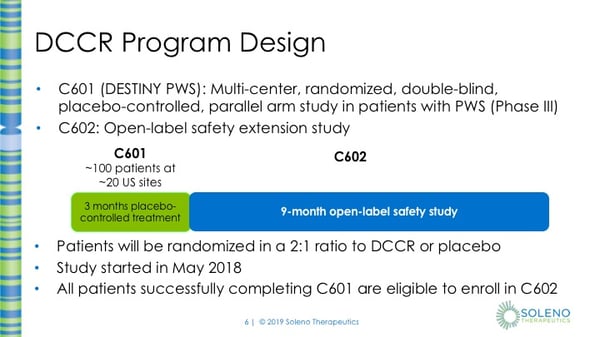 Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
 Literature Search Flowchart Abbreviations Oct Open Label Clinical
Literature Search Flowchart Abbreviations Oct Open Label Clinical
 Open Label Randomized Clinical Trial Of Atropine Bolus Injection
Open Label Randomized Clinical Trial Of Atropine Bolus Injection
 Prospective Open Label Clinical Trial Of Trihexyphenidyl In Children
Prospective Open Label Clinical Trial Of Trihexyphenidyl In Children
 Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
Pws Clinical Trial Webinar Diazoxide Choline Controlled Release Dccr
 Open Label Clinical Trials Of The Clinical Efficacy Of Nicotine On
Open Label Clinical Trials Of The Clinical Efficacy Of Nicotine On
Efficacy And Safety Of Once Weekly Semaglutide Versus Exenatide Er
 Drug Trials Learning Outcome Describe The Use Of Open Label Blind
Drug Trials Learning Outcome Describe The Use Of Open Label Blind
 Consort Flowchart For A Single Arm Open Label Phase 2 Clinical
Consort Flowchart For A Single Arm Open Label Phase 2 Clinical
 Table 2 From Phase I And Ii Clinical Trials Of Trastuzumab
Table 2 From Phase I And Ii Clinical Trials Of Trastuzumab
 Dna Priming And Influenza Vaccine Immunogenicity Two Phase 1 Open
Dna Priming And Influenza Vaccine Immunogenicity Two Phase 1 Open
Clinical Trials In Orthopedic Disorders Table Of Contents
Research Announcements Prader Willi Syndrome Association Usa
 Cll Sll Clinical Trial Study Design Venclexta Rituximab
Cll Sll Clinical Trial Study Design Venclexta Rituximab
 Relapsing Multiple Sclerosis Rms Treatment Ocrevus Ocrelizumab
Relapsing Multiple Sclerosis Rms Treatment Ocrevus Ocrelizumab
 Breast Clinical Trials Adjuvant Her2 Bre 186 Cohort A B Closed
Breast Clinical Trials Adjuvant Her2 Bre 186 Cohort A B Closed
 Lilly Trials On Twitter Open Label Design Usually Applies To Non
Lilly Trials On Twitter Open Label Design Usually Applies To Non
 Evaluation Of Autologous Adipose Derived Mesenchymal Stem Cells Ad
Evaluation Of Autologous Adipose Derived Mesenchymal Stem Cells Ad
 Ventavis Iloprost Clinical Trials
Ventavis Iloprost Clinical Trials
Souvenir Ii Open Label Extension Clinical Trials Souvenaid
 Fillable Open Label Study Edit Online Download Samples In Word
Fillable Open Label Study Edit Online Download Samples In Word
 The Refresh Trial On Twitter An Open Label Randomized Clinical
The Refresh Trial On Twitter An Open Label Randomized Clinical
 Pdf An Open Label Randomized Clinical Trial Comparing The Safety
Pdf An Open Label Randomized Clinical Trial Comparing The Safety
Plos Medicine Safety And Pharmacokinetics Of The Fc Modified Hiv 1
 Catabasis Pharmaceuticals Reports Positive Results From Open Label
Catabasis Pharmaceuticals Reports Positive Results From Open Label
 Study Design Of Clinical Trial Open Label Multiple Dose
Study Design Of Clinical Trial Open Label Multiple Dose


0 Response to "Open Label Clinical Trial"
Post a Comment